我们使用机器学习技术将英文博客翻译为简体中文。您可以点击导航栏中的“中文(简体)”切换到英文版本。
使用亚马逊管理区块链为治疗药物的开发做出贡献
在制药行业,临床研究和临床试验的成本极高,这导致药品价格和保险费用上涨。此外,高昂的成本导致国家研究机构和大学的临床试验数量减少,减缓了药物研究的进展。此外,学术论文和
为了应对这些挑战,医疗保健行业因其防篡改和透明特性而扩大了对区块链的使用。新型冠状病毒感染进一步扩大了区块链在药品分销、假冒产品对策和临床试验中数据利用的使用。
通过在该系统 中使用
根据内阁办公室的沙盒计划,Susmed使用区块链技术对临床数据监控系统进行了演示。Susmed首席技术官本桥友光解释了以管理区块链为中心的区块链架构以及对区块链对服务的需求如下:
- 区块链技术使得篡改数据变得困难,这有助于监测临床试验和某些临床研究。
- 与传统方法相比,区块链提高了安全水平,同时实现了经济高效和准确的监控。这降低了研发成本,有助于维持和增强日本制药业的国际竞争力以及社会保障的可持续性。
该演示是与日本国家癌症中心合作进行的,并在一项临床试验中进行了监测,该试验旨在制定一项锻炼计划,以促进没有运动习惯的癌症幸存者的行为改变。
通过直接在区块链上记录传输的数据,可以持续确保数据的可靠性。这使得通过使用防止客户端和中继服务器漏洞的技术来伪造存储的数据变得很困难,包括使用专门的应用程序进行数据输入(不从源数据转录到报告数据)。因此,即使监控人员没有访问该网站并对照可报告的数据检查源文件,数据的可靠性也是可以验证的。
此后,卫生、劳动和福利部长以及经济、贸易和工业大臣以书面形式宣布,法律允许区块链技术取代药品和医疗器械临床试验所需的监测。
Susmed 还为这种方法申请了专利,我们相信这是区块链技术的一项全新而有价值的应用。Susmed 亚马逊云科技 的原则如下:
- 尽可能使用完全托管的服务,通过使用无服务器配置来降低运营成本
- 利用 亚马逊云科技 CodeBuild 进行持续集成
- 由于 亚马逊云科技 服务具有高度的可靠性,除非有其他重要理由,否则请使用 亚马逊云科技 服务
基于该政策,我们最初在
选择使用区块链技术
在临床研究和试验中,需要对多个利益相关者进行审计,这可能需要大量的时间和金钱。根据试验的性质,费用可能超过数亿美元(日元),其中20-50%外包给CRO(合同研究组织)或SMO(临床试验设施支持组织)。
下图说明了参与临床研究和临床试验的各方及其各自的关系(摘自日本SMO协会)。

如果伪造测试或研究结果,不仅会浪费在审计和测试上花费的大量时间和金钱,而且药物也将无法得到适当的评估,从而无法为医疗保健专业人员和患者提供适当的医疗服务。
如今,观察者和其他人工操作员证实,大多数数据都具有很高的防篡改性,这是区块链技术获得关注的主要原因。
区块链仅被定位为安全层之一。目标不是实现权力下放,而是将信息技术权限结构从分层结构转变为平行结构。
至于区块链网络,它们可以大致分为三种类型:
- 公共 ——任何人都可以使用和查看的区块链
- 私 有 — 区块链,其中特定的个人或公司是唯一的管理员,只有授权用户才能查看和使用写入私有链的交易信息
- 联盟 — 多个授权组织或公司共同拥有控制权,可以在参与者之间就交易信息达成共识
对于临床研究和临床试验,我们决定使用联盟型区块链,该区块链可以识别可以加入区块链并使用多数投票的参与者,原因如下:
- 它不会生成大量数据,也不会施加严格的吞吐量要求
- 无需将数据分发给不确定数量的人进行共享和验证
数据库与区块链的比较
出于防篡改目的,也可以将数据库视为一种选择。但是,很难证明数据存储在数据库中或仅使用数据库技术与多家公司共享后没有人篡改。
与区块链不同,数据库是由管理员操作的集中式账本。由于数据库的用户具有管理员权限,因此很容易在不留下任何证据的情况下篡改数据。因此,没有透明度。就数据库而言,可能会泄露数据,具体取决于管理员的权限设置,但是数据库用户很难验证该数据的完整性。区块链的一个关键特性是,任何拥有正确工具的人都可以验证写入其中的数据。在这方面,使用区块链技术的临床试验也是解决医疗问题的开创性努力。
美国国家标准与技术研究所(NIST)发布的一份报告和一份名为 “你需要区块链吗?” 的白皮书详细说明了使用区块链的重要性(CVCBT,2018)。白皮书中的下图显示,如果利益相关者值得信赖(没有动力进行篡改),那么常规数据库就足够了,但在其他情况下,区块链值得使用。

解决方案概述
有关整体架构,请参阅以下 2020 年 10 月 亚马逊云科技 开发日上的
在下图中,我们描述了向区块链写入数据之前的架构。
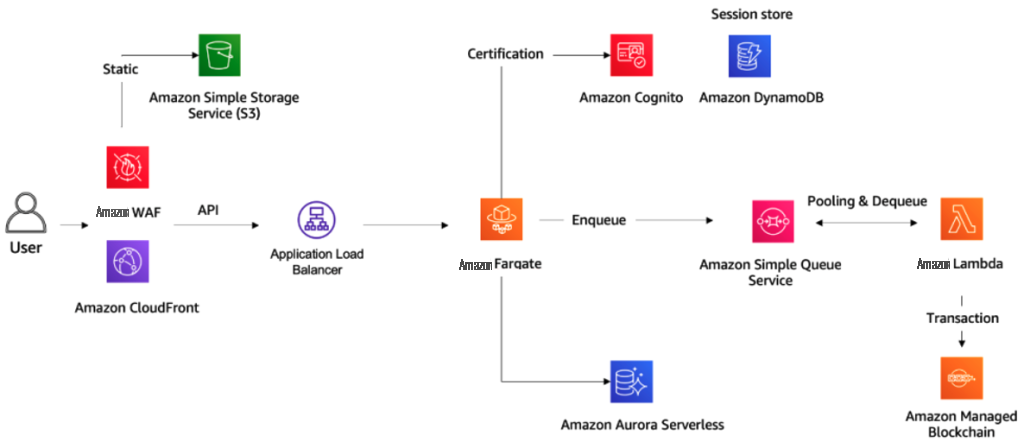
让我们讨论一下该架构背后的三个主要设计原则。
区块链应根据需要与组件相结合
如前所述,区块链具有高度防篡改的优势。但是,必须谨慎对待配置。仅靠区块链无法满足所有要求。根据所使用的链,它可能与需要高吞吐量或复杂交易的工作负载不兼容。(有关更多信息,请参阅
使用无服务器配置减轻运营负担
我们尽可能使用完全托管的服务。我们还采用了无服务器架构来降低运营成本。所有基础架构配置均使用自动配置工具 (Terraform) 进行管理,因此部署相同的环境不需要花费大量时间。在没有运营负担和自动化的情况下,我们能够使用七名工程师和一名设计师开发和运营三项服务,并建立高效的开发系统。
随时可用,随时可以销毁
尤其是在临床试验系统中,人们总是决定试验将在一定时间内完成。因此,云计算和Susmed的服务非常兼容,因为它们可以在需要时使用,不需要时可以删除资源。此外,在区块链中,创建和删除资源需要花费大量精力,但是在托管区块链中,您可以创建和删除整个区块链资源本身,这样可以轻松灵活地报废和构建。
结论
在这篇文章中,我们讨论了Susmed如何通过使用新的数字技术——区块链——来解决临床试验的高成本和效率低下的问题,以及由此导致的社会保障成本增加和研究延迟的问题。亚马逊云科技 提供 200 多种云服务,包括托管区块链,自 2011 年以来,亚马逊云科技 一直坚定地致力于在日本进行持续创新和投资。亚马逊云科技致力于通过云计算实现业务转型和解决国家挑战来为社会做出贡献,我们将继续支持云端的医疗保健数字化转型,并与Susmed、Aculys Pharma以及许多其他正在采取渐进措施解决医疗行业问题的公司一起进一步加速日本的数字化转型。我们将继续在整个日本加速数字化转型。
这篇文章是从 亚马逊云科技 日语博客翻译而来的,
作者简介
本桥友光 是Susmed 的首席技术官。
丰山公宏 是 亚马逊云科技 日本的业务开发经理。
中武由纪 是 亚马逊云科技 日本的解决方案架构师。
*前述特定亚马逊云科技生成式人工智能相关的服务仅在亚马逊云科技海外区域可用,亚马逊云科技中国仅为帮助您发展海外业务和/或了解行业前沿技术选择推荐该服务。